Key Takeaways
- Solid state polymerization makes polyester stronger by increasing its size.
- This process helps polyester handle heat better in hot places.
- It is eco-friendly, using 44% less energy and cutting CO₂ by 26%.
- This method is important for making textiles and packaging better.
- Knowing about this process shows how useful and tough polyester is.
What Is the Solid State Polymerization Process for Polyester?
Definition of Solid State Polymerization
Solid-state polymerization improves polyester by making it stronger. It heats small pellets below melting to boost molecular weight. This keeps the material solid and prevents melting or damage. By controlling heat and conditions, polyester becomes tougher and better performing.
Purpose and Importance in Polyester Production
This process is key to making strong and durable polyester. It builds the right molecular structure for strength and heat resistance. Industries like textiles, packaging, and engineering need this high-quality polyester. Without it, polyester wouldn’t work well for tough jobs.
Key Characteristics of the Solid State Polymerization Process
Solid-state polymerization has special features that make it work well. It uses heat between the glass transition and melting points, around 165 ± 50°C. The process happens in nitrogen gas or vacuum to stop damage. Pellets are crystallized first to avoid sticking together. It can be done in batches or continuously, based on needs. These steps ensure polyester meets high standards.
| Step/Condition | Description |
|---|---|
| Starting Material | Clear pellets (0.57-0.62 IV) made by regular methods. |
| Temperature Range | Done between glass transition and melting points of polyester. |
| SSP Temperature | Kept at 165 ± 50°C, ideally ± 40°C, better at ± 25°C, best at ± 15°C for PET crystallization. |
| Inert Environment | Done in nitrogen gas or vacuum to stop damage. |
| Pre-Crystallization Step | Pellets are changed to a crystalline form to stop clumping. |
| Reactors/Processing Stations | Uses tools like a crystallizer and pre-heater for processing. |
This process also changes how the material stretches and flows. Higher drying heat makes polyester thicker. Nitrogen stops it from breaking down. These details show why careful control is so important in this process.
Step-by-Step Solid State Polymerization Process for Polyester
Pre-Polymer Preparation
The first step is getting the pre-polymer ready. Start with clear polyester pellets made by melting. These pellets have an IV of 0.57 to 0.62. Special catalysts and heat are very important here. For example, TIS catalyst speeds up reactions. DBTO helps make the pellets more crystalline. Heating at 205°C for five hours improves strength and heat resistance. This step makes the pellets ready for the next process.
| Variable | Effect on Polymerization Results |
|---|---|
| PVP Concentration | More PVP lowers resistance until a limit is reached. |
| AgNO3 Concentration | Too little gives poor conductivity; too much causes problems. |
| Reaction Time | Short time lowers resistance; too long increases it again. |
| UV Exposure | Helps polymerization and improves fabric properties. |
Crystallization of Polyester Pellets
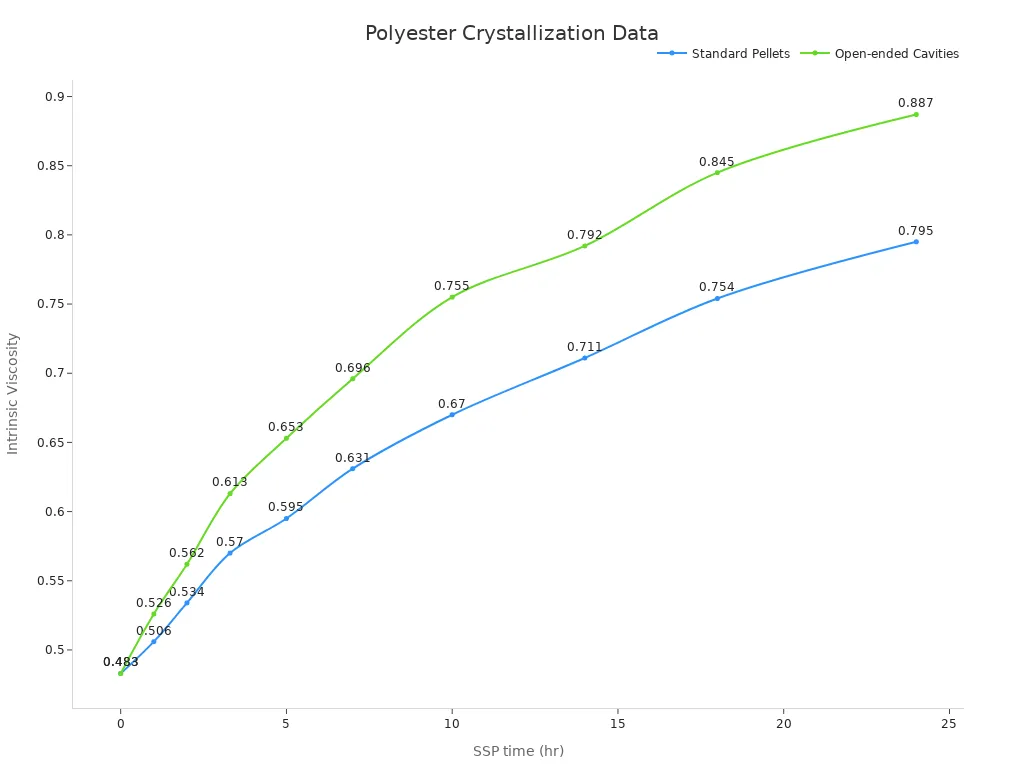
Next, the pellets are crystallized to stop them from sticking. Cold crystallization is used for this step. This process is watched closely to get the right IV. For instance, after seven hours, the IV rises from 0.4830 to 0.631. This step is key for keeping the product consistent and high-quality.
| SSP time (hr) | I.V. of Standard Pellets | I.V. of Open-ended Cavities |
|---|---|---|
| 0 | 0.4830 | 0.4830 |
| 1 | 0.506 | 0.526 |
| 2 | 0.534 | 0.562 |
| 3.3 | 0.570 | 0.613 |
| 5 | 0.595 | 0.653 |
| 7 | 0.631 | 0.696 |
| 10 | 0.670 | 0.755 |
| 14 | 0.711 | 0.792 |
| 18 | 0.754 | 0.845 |
| 24 | 0.795 | 0.887 |
Heating Above Glass Transition Temperature
After crystallizing, the pellets are heated above their glass transition temperature (Tg). For PET, Tg is between 69°C and 80°C. Heating above Tg makes the pellets soft and flexible. This step dries the pellets and gets them ready for polymerization. It also keeps the material strong while improving its molecular weight. By controlling heat and time, the polyester becomes better and stronger.
Nitrogen Purging or Vacuum Conditions
To make strong polyester, the right environment is key. This step uses nitrogen purging or vacuum conditions. Both methods remove oxygen and moisture that can harm polyester. Nitrogen purging fills the reactor with a safe gas to stop oxidation. Vacuum conditions lower pressure to get rid of unwanted gases and impurities.
These controlled settings make the reaction work better. They also stop side reactions that could weaken the polyester. Keeping these conditions helps the material become stronger and last longer. This step is crucial for making polyester that meets industry needs.
Solid-State Polycondensation Reactions
The main part of the process is solid-state polycondensation. Here, polyester pellets are heated under strict control. The heat makes polymer chains grow longer, raising molecular weight. This step makes the polyester stronger and more heat-resistant.
The reaction speed depends on temperature and time. Higher heat makes reactions faster but needs careful watching to avoid damage. The table below shows important data about reaction speed and results:
| Evidence Type | Description |
|---|---|
| Molecular Weight Increase | Higher temperature and time raise the molecular weight of PEF. |
| Kinetic Rate Constants | Models show reaction speeds for esterification and polycondensation. |
| End-Group Variation | Carboxyl and hydroxyl end-groups change during different SSP reactions. |
Knowing these details helps improve the polycondensation process for better outcomes.
Cooling and Final Additive Integration
After reactions finish, the polyester pellets must cool. Cooling makes the material stable and keeps its new properties. It also stops heat from damaging the polyester.
Once cooled, final additives can be added to improve features. For example, UV stabilizers protect against sunlight, and flame retardants add safety. These additives make polyester useful for many things, like clothes and engineering products.
By carefully handling this last step, the polyester meets quality and performance goals.

Benefits of Solid State Polymerization for Polyester
Better Molecular Weight and Strength
Solid-state polymerization makes polyester stronger by increasing its molecular weight. This process stretches the polymer chains, making the material tougher. Stronger polyester resists damage and lasts longer. It works well for tough jobs like industrial fabrics and strong packaging. This process ensures polyester stays durable under heavy use.
Improved Heat and Strength Properties
This process also improves how polyester handles heat and pressure. It makes the material stronger and more heat-resistant. For example:
- PBSeT-PLA copolyesters break down at higher temperatures.
- P(X)’s softening temperature rises by 14 °C to 128.6 °C at P2.
- Strength improves a lot: tensile strength by 32.8%, puncture load by 38.5%, and tearing strength by 71.8% at P2 compared to P0.
These changes make polyester great for advanced uses like engineering parts and special packaging.
Saves Energy and Helps the Environment
Solid-state polymerization is good for the planet. It uses less energy and reduces pollution. Key improvements include:
| Metric | Percentage Reduction |
|---|---|
| Energy Use | 44% |
| CO₂ Emissions | 26% |
| Water Use | 31% |
| Chemical Use | 12% |
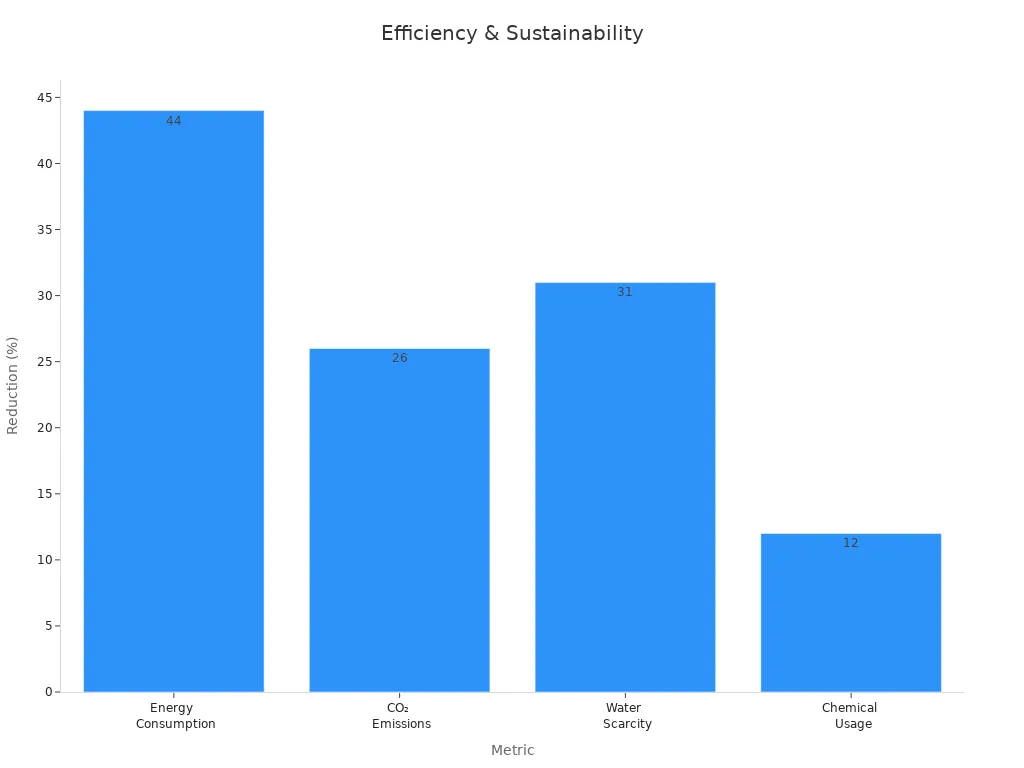
Using this process helps protect the environment. It saves resources and lowers waste. Eco-friendly manufacturers prefer this method. Solid-state polymerization makes polyester production greener while keeping quality high.
Uses of High-Performance Polyester Products
Solid-state polymerization helps make strong and durable polyester. This process improves how polyester handles heat and tough conditions. It is used in industries that need reliable and high-quality materials. Many products benefit from this advanced way of making polyester.
Important Market Facts
The polyester fiber market is growing fast, especially for tough uses. The table below shows key details about the market:
| Aspect | Details |
|---|---|
| Market Size | Big growth in polyester fiber, focused on strong uses. |
| Application Segments | Used in clothes, home items, and industrial fabrics. |
| Competitive Landscape | Led by big companies like Reliance Industries and Indorama Ventures. |
| Challenges | Changing material costs and eco issues affect the market. |
| Innovations | New methods improve quality and lower costs. |
| Revenue Share (2023) | PSF at 65.5%, clothing at 47.6%. |
Different Uses
Solid-state polymerization makes polyester useful in many ways:
- Polyester staple fiber (PSF) made up 65.5% of market money in 2023.
- Clothes made with polyester brought in 47.6% of market money.
- Virgin polyester had 86.5% of the market because it is high-quality.
Reasons for Demand
Several things make people want high-performance polyester:
- Better fiber technology makes stronger and more useful products.
- Online shopping needs strong polyester for packaging.
- More middle-class people buy polyester products.
- Problems like microfiber waste and cost changes need smart fixes.
Solid-state polymerization helps polyester meet these needs. It makes materials for tough jobs like industrial fabrics, home goods, and special packaging. This process creates top-quality polyester that works very well.
The solid-state polymerization process is key in making polyester today. It creates strong, long-lasting, and eco-friendly materials. This process helps polyester meet the needs of industries like clothing, packaging, and engineering.
Learning about this process shows how it changes polyester. It’s not just about making polyester but creating better, longer-lasting products for many uses.
